During late September, the developer uniQure announced the results of its first successful treatment of Huntington’s disease via gene therapy. Although the treatment still hasn’t been approved by the FDA, the first round of trials showed that the treatment slowed down the progression of the disease by 75%.
The study consisted of 26 participants each given either a low or high dose. Three years after the treatment was administered, the participants provided the higher dose demonstrated 75% slower progression of the disease. The treatment would give patients decades more of “good quality life,” according to what the director of the UCL Huntington’s Disease Center Sarah Tabrizi told BBC.
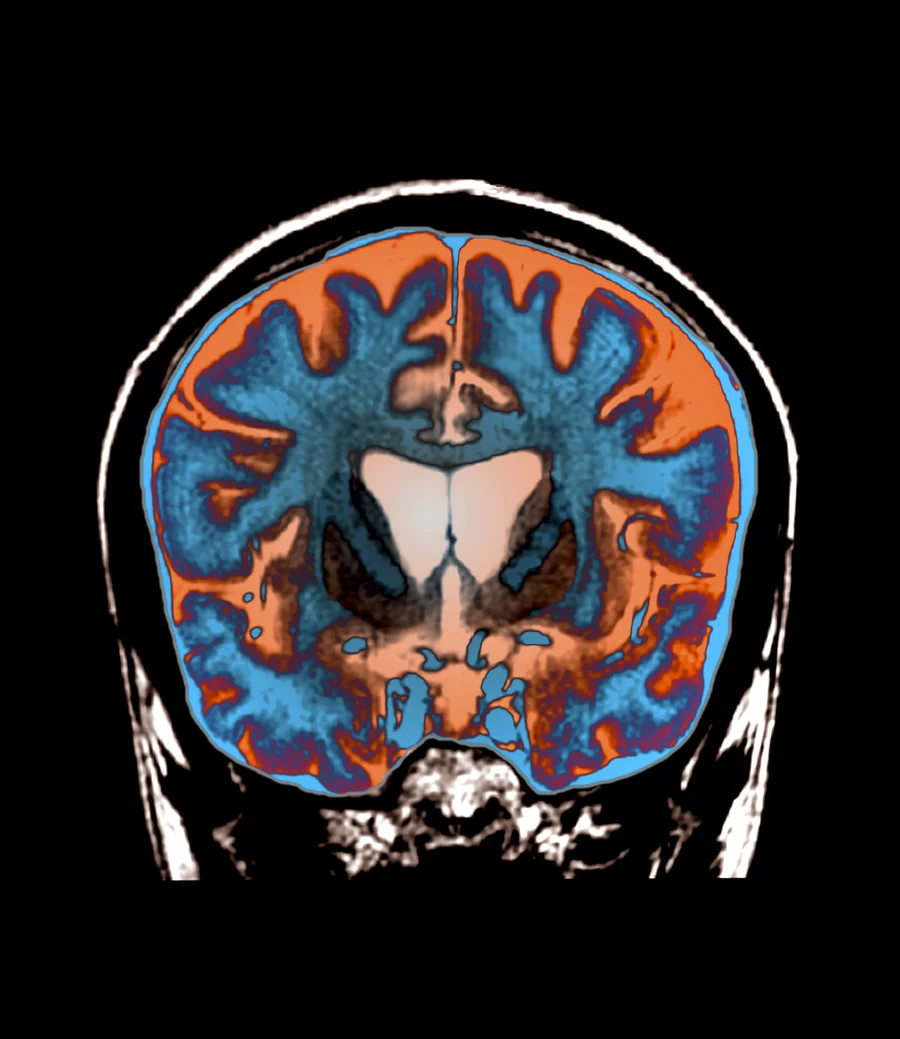
Huntington’s disease is a rare neurodegenerative disorder that affects motor function leading to physical and mental deterioration. Patients usually start exhibiting symptoms around middle age and typically live 10-30 years after diagnosis. The disease is caused by a mutation of the HTT gene and multiplication of a faulty Huntington’s protein. All attempts at finding a treatment have failed and devastated the community until now. “I cried with every single patient. It just was this crazy feeling that, for the patients and families, almost can’t feel real,” said director of the UAB Huntington’s Disease Clinic, Victor Sung.
The treatment is called AMT-130 and is implemented via an 8-10 hour surgery. The treatment stops the production of the deadly mutant protein that causes Huntington’s disease. “Since the HD gene was first sequenced over 30 years ago, we have been on a quest to try to slow or stop this difficult disease, and these preliminary results are finally a huge step in that direction,” stated Victor Sung.
Since this is only the first trial of the drug, it has yet to be ratified by the FDA. UniQure aims to get it approved before 2026 without needing a third round of trials. “We certainly remain cautious about interpretation of these results since they are only in a small number of patients, but the 75 percent disease-slowing is a big number and still very much a reason to be excited,” said Sung.